Custom-printed structural spacers designed for large-segment bone loss due to high-energy trauma, oncology, or chronic infection.
Client:
Advanced Limb Salvage Center
Date:
January 2026
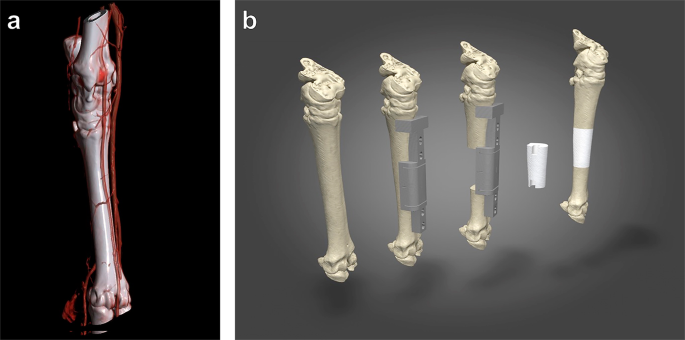
Segmental bone defects, often defined as voids greater than 2cm or those exceeding 50% of the bone diameter, do not heal through natural physiological processes. These "critical-sized" defects, resulting from radical tumor resection, severe comminuted fractures, or debridement of osteomyelitis, traditionally required aggressive bone grafting or long-term external fixation. The Segmental Bone Reconstruction application utilizes 3D-printed titanium spacers to provide immediate mechanical stability and a biological bridge for long-term limb salvage.
Unlike solid metallic rods, which can lead to stress shielding and subsequent bone atrophy, our segmental spacers are engineered with a gradient of porosity. This ensures that the implant shares the physiological load with the surrounding native bone, promoting health rather than resorption.
A successful segmental reconstruction depends on the biological "handshake" between the metal and the host bone. This application utilizes Laser Powder Bed Fusion (LPBF) to create a dual-surface environment.
The ability to print "on-demand" anatomical spacers has transformed the surgical timeline for limb salvage. In oncology cases, the spacer can be designed and printed during the pre-operative planning phase, allowing for immediate reconstruction following tumor resection.
Key Clinical Advantages:
Every segmental reconstruction device undergoes rigorous finite element analysis (FEA) to ensure it can withstand the repetitive cycles of human ambulation. By adhering to strict FDA regulatory pathways and leveraging evidence-based innovation, this application provides a validated, high-fidelity solution for the most complex bone reconstruction challenges in modern surgery.
.png)
Join a trusted ecosystem dedicated to the advancement of 3D-printed implant technologies. We transform complex anatomical challenges into clinical successes through evidence-based innovation and regulatory transparency.