Patient-specific titanium solutions, including talus spacers and bone segment replacements, designed for complex anatomical defects.

A dedicated hub for multi-centered clinical studies measuring bone in-growth, implant performance, and long-term surgical success.

Expert resources on FDA regulatory pathways and device design considerations to support informed adoption of next-gen technologies.
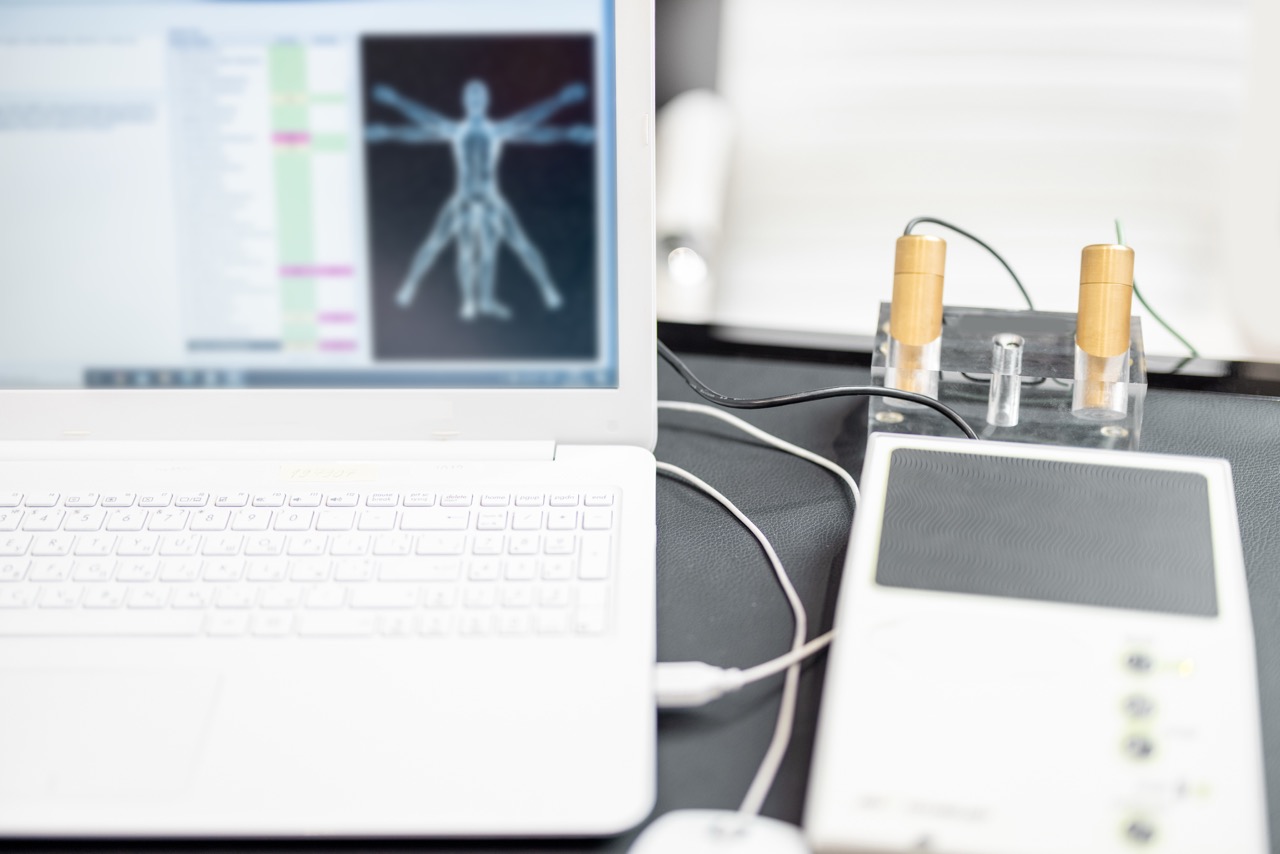
Centered on the advancement of additive manufacturing, we combine regulatory awareness, clinical research insight, and orthopedic education to support informed surgical adoption.

We combine advanced additive manufacturing with rigorous clinical evaluation to validate the performance of 3D-printed orthopedic solutions.
Regulatory Pathways Evaluated
Evidence-Based Clinical Adoption
Multi-Centered Clinical Studies
Focused on Patient Outcomes
"Additive Orthopaedics has completely elevated our understanding of 3D-printed technologies. Their commitment to clinical validation and regulatory transparency has helped us navigate the most complex reconstructive challenges with confidence."
Dr. Lawrence S., Orthopedic Surgeon
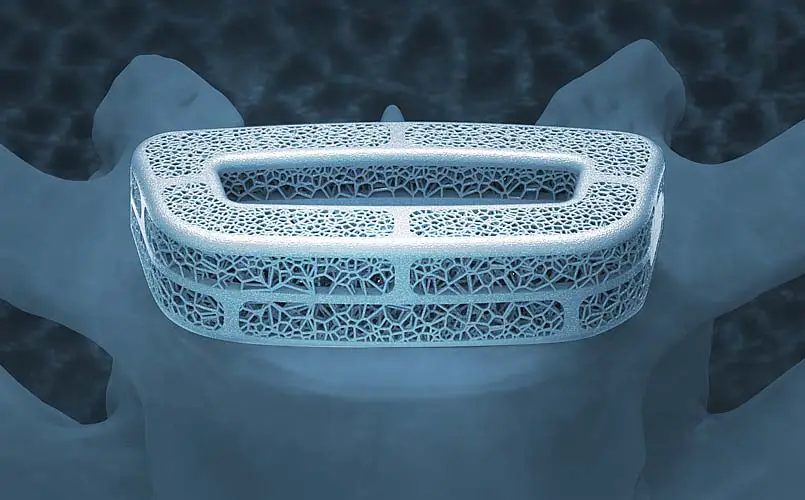
We prioritize deep insights into FDA regulatory pathways and device design considerations to ensure safe, informed adoption of next-gen technology.
We lead the advancement of 3D-printed orthopedic technologies through multi-centered studies that measure bone in-growth and long-term performance.
We serve as a trusted knowledge hub for healthcare stakeholders, promoting the understanding of additive manufacturing in modern musculoskeletal care.
.png)
Join a trusted ecosystem dedicated to the advancement of 3D-printed implant technologies. We transform complex anatomical challenges into clinical successes through evidence-based innovation and regulatory transparency.