A modular solution for severe acetabular defects and pelvic discontinuity utilizing proprietary porous scaffolds.
Client:
Orthopedic Research Institute
Date:
December 2025
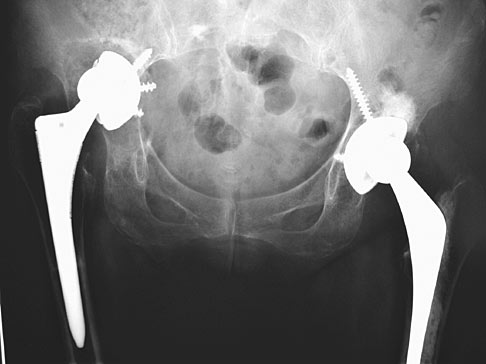
Reconstructing the hip in the presence of massive acetabular bone loss remains one of the most formidable challenges in revision arthroplasty. Traditional hemispherical cups often fail to achieve stability when Paprosky Type III defects or pelvic discontinuities are present. The Acetabular Revision System leverages advanced additive manufacturing to bridge these voids, providing a structural scaffold that facilitates biological fixation where conventional implants cannot.
The core of this system is our proprietary Tri-Lattice Porous Structure. Unlike traditional coatings that only provide surface-level roughing, this system is printed as a monolithic unit with fully interconnected porosity.
No two pelvic defects are identical. This application allows for a modular approach, combining standardized revision cups with patient-specific augments.
The Acetabular Revision System is manufactured using Laser Powder Bed Fusion (LPBF) with medical-grade titanium (Ti-6Al-4V). This ensures the device can withstand the high-cycle fatigue loads of a weight-bearing joint while maintaining a lightweight profile.
Key Clinical Advantages:
Current clinical studies focused on 3D-printed porous titanium in the pelvis indicate high rates of success in limb salvage and complex revision. By adhering to rigorous FDA regulatory pathways and focusing on evidence-based innovation, this system provides a validated solution for the most difficult hip reconstructions in modern orthopedics.
.png)
Join a trusted ecosystem dedicated to the advancement of 3D-printed implant technologies. We transform complex anatomical challenges into clinical successes through evidence-based innovation and regulatory transparency.